Accelerating Clinical Trial Operations with Generative AI and Azure Cloud: A Modern Case Study

Overview
In the world of biopharmaceuticals, time is of the essence. With the ever-growing complexity of clinical trials and regulatory compliance, organizations face increasing pressure to reduce delays and bring lifesaving therapies to patients faster. In this case study, we explore how a global biopharma organization leveraged Microsoft Azure and Azure OpenAI Service to optimize clinical trial site activation, improve predictive modeling, and modernize data operations—achieving a site activation time reduction of approximately 10%.
Executive Summary
This organization set out to enhance operational efficiency and accelerate clinical development by:
Implementing a unified, cloud-native data platform on Microsoft Azure
Integrating Azure OpenAI Service to support generative AI-powered applications
Reducing manual processes related to site selection and documentation
Improving data-driven forecasting for better decision-making
Key Outcomes:
~10% faster clinical trial site activation
Enhanced employee engagement through AI-driven tools
Faster insights for predictive modeling and trial optimization
Challenges
Clinical trials involve extensive coordination, manual documentation, and complex data integration. Historically, selecting suitable research sites and managing trial logistics were labor-intensive and time-consuming, delaying trial start dates and increasing costs. The organization needed a scalable solution to streamline site activation and predictive operations.
Proposed Solution Architecture
Technology Stack
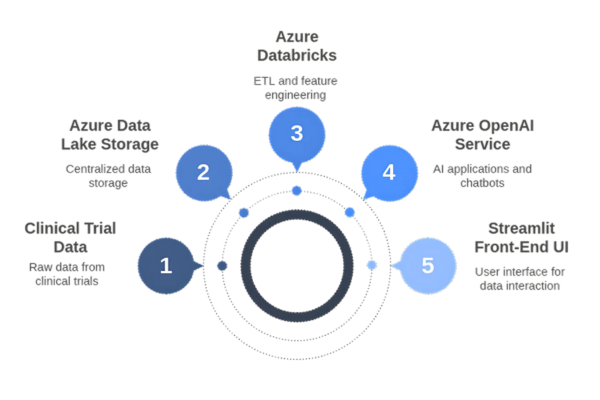
Applications Enabled by the Solution
1. AI-powered Site Selection Tool: Generates initial trial site lists using LLM-based reasoning and historical trial data
2. Predictive Analytics Dashboard: Visualizes risk forecasts and potential delays across trial sites
3. AI Chatbot for Internal Teams: Enables natural language querying of trial data, site performance, and historical KPIs
4. Real-Time Engagement Assistant: Supports medical liaisons in discussing protocol adjustments and trial design scenarios with stakeholders
5. Document Summarizer and Generator: Automatically drafts and refines SOPs, consent forms, and regulatory documents using generative AI
Implementation Timeline
Month 0-3: Data estate modernization and migration to Azure
Month 4-6: Development of unified data pipeline and analytics workflows
Month 7-9: Integration of generative AI capabilities and Streamlit UI
Business Impact
1. Accelerated Site Activation
Initial site recommendations reduced from weeks to 48 hours
Full site selection cycle reduced by ~10%
2. Enhanced Predictive Modeling
Clinical teams can anticipate delays early
Forecasts refined using historical and real-time trial data
3. Smarter Workflows and Engagement
Generative AI chatbots allow team members to query complex datasets
Real-time data walkthroughs improve client engagement and reduce rework
Future Plans
The success of this initiative serves as a blueprint for future AI-led transformations. The organization is now exploring:
Generative AI assistants for protocol design
Automated monitoring and reporting for compliance
Chat-based interfaces for internal knowledge management
Conclusion
This case demonstrates how modern biopharma operations can benefit from an AI-first architecture using Azure and generative AI tools. By automating manual workflows and accelerating clinical processes, organizations can drive innovation, reduce costs, and deliver therapies to market faster.
Ready to Accelerate Your Clinical Trials?
Huebits helps life sciences organizations modernize trial operations using AI-driven solutions on Microsoft Azure. From faster site selection to smarter forecasting, we build scalable systems tailored to your needs.
Want a solution like this for your organization?
Let’s explore what AI can do for you.
